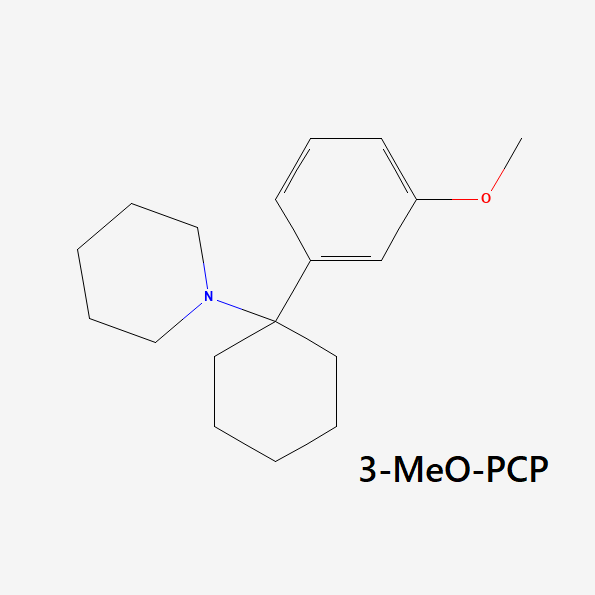
3-MeO-PCP (AKA 3-Methoxyphencyclidine)
was first synthesized in 1979 as part of some early research comparing the effects of PCP and its analogs. It didn’t enter the market as a research chemical until many years later, in 2011.
This compound is very potent, even stronger than PCP, 2-MeO-PCP, and 4-MeO-PCP.
Unfortunately, it also has a high likelihood of inducing hypomanic states. Some people like this effect, but it can be scary for others. People looking for a “chill” arylcyclohexylamine should avoid this one. Even low doses appear to carry a strong sense of delusional thought patterns and stimulation.
Some early reports suggested this drug also had opioid-like activity — users would feel physically numb and relaxed, with an underpinning sense of euphoria. However, researchers have tested this theory and compared the affinity of this drug on the μ-, δ-, or κ- opioid receptors but found no activity at all [11]. It’s likely that the strong dissociative action is what is offering an almost opioid-like quality — users feel disconnected from the body, which inherently offers some painkilling effects.
3-MeO-PCP was found to have a high binding affinity (Ki) for the MK-801 binding site of the NMDA receptors (20–38.1 nM) and sigma 1 receptors (42 nM), moderate affinity for the serotonin transporter (216–1571 nM), low activity on the norepinephrine transporter (1,808 nM), and very low or no activity at the sigma 2 receptors and dopamine transporters [5,11].
4-MeO-PCP
was one of the first compounds to enter the market during the new wave of online designer drug markets. The chemical structure was just different enough for vendors to sell the material without directly breaking any laws.
This compound was first sold online in 2008, and its success sparked an entire industry of designer PCP and ketamine analogs which continue to come out every year [4]. With that said, the first publication of 4-MeO-PCP synthesis dates back to the 1960s [14]. It remained on the shelf for decades until someone realized they could sell the compound pseudo-legally as a PCP alternative.
4-MeO-PCP is less potent than PCP and 3-MeO-PCP but still carries substantial dissociative and hallucinogenic properties in doses of around 40 mg.
The receptor Ki values for 4-MeO-PCP are reported at 620 nM for the NMDAR, 1,811 for the DAT, 1,811 for the NET, and 900 for the SERT.
How Do Arylcyclohexylamines Work?
Arylcyclohexylamines interfere with the function of glutamate — a key neurotransmitter involved with brain function (and, therefore, consciousness). This results in a powerful dissociation of the mind from the body — all without extinguishing consciousness.
Most of the arylcyclohexylamine class of drugs work by binding to the PCP binding site of the NMDA receptor [8]. PCP was the first compound ever studied and was instrumental in understanding the NMDA receptors. However, most members of this group — not just PCP — bind to this same receptor.
Once bound, arylcyclohexylamines block the effects of NMDA, resulting in their characteristic dissociative effects.
Arylcyclohexylamines also interact with the dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT) to exert additional effects on mood and cognition.
For example, PCP is a strong stimulant in addition to its dissociative qualities. This effect stems from the compound’s affinity for dopamine, norepinephrine, and serotonin transporters. By blocking the reuptake of these neurotransmitters, these compounds exert a potent amphetamine-like effect — boosting cognition, increasing vigilance and alertness, and raising body temperature.
One study found PCP was 8 times more potent than D-amphetamine and 34 times more potent than methylphenidate at inhibiting serotonin (5-HT) uptake [12].
PCP has also been found to interact with the sigma receptors — especially the sigma 2 receptor — which are involved with a wide range of neurological functions. The specific function of these receptors is not well understood. Several psychoactive drugs have been found to interact with these receptors in addition to PCP and PCP-analogs, including cocaine, morphine, fluvoxamine, methamphetamine, and DXM (dextromethorphan).
The binding affinity for each arylcyclohexylamine varies — some have a stronger dopamine component; others are stronger NMDA antagonists. These changes in binding affinity impact the drugs’ subjective effect profile — making them stronger dissociatives, sedatives, or stimulants than other members of the group.
The arylcyclohexylamine class is also notoriously biphasic in terms of their effects — this means they produce different effects in low doses than they do in higher doses. In general, lower doses are stimulating, while higher doses are sedative and cataleptic. Most members of this group share these qualities.
N-Methyl-D-Aspartate (NMDA) Receptors
The NMDA receptors play a key role in synaptic plasticity, memory, and pain perception [42, 43]. These receptors are activated by glutamate, which is the primary excitatory neurotransmitter in the brain.
A variety of psychoactive drugs rely on an interaction with the NMDA receptors to exert their effects, including alcohol, GHB, xenon gas, nitrous oxide, methadone, and all the psychoactive arylcyclohexylamines. Blocking these receptors or otherwise inhibiting their function leads to a state of dissociation and inhibits the formation of memories. It also results in the characteristic dissociation and depersonalization inherent to this class — but the specific mechanism for how this works is still not well understood.
Reviews
There are no reviews yet.