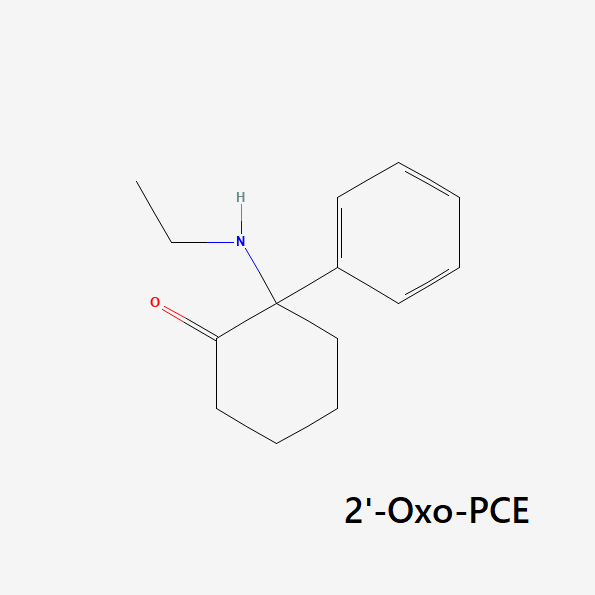
O-PCE (2-Oxo-PCE, Eticyclidone)
2-Oxo-PCE (AKA eticyclidone or O-PCE) is most similar to DCK — the only difference is that O-PCE has a one-carbon chain, while DCK has a 2-carbon chain in the same position. This difference is subtle but produces distinct changes in the effect profile.
Compared to DCK and ketamine, O-PCE is significantly more stimulating and has fewer inhibitory effects on the senses. This compound doesn’t normally exert its dissociative effects until 3 or 4 hours into the trip as it begins to wear off.
Because of the stimulating nature of this compound, O-PCE is often referred to as “chaotic” and “wonky,” — producing unexpected urges and bizarre behavior — especially in higher doses. In low to moderate doses, it’s usually described as warm and euphoric, with less of the heavy dissociative effects of other drugs like DCK or ketamine.
The receptor Ki values for O-PCE have not yet been reported.
Arylcyclohexylamine Summary
- Chemical Structure: Contains a cyclohexyl group fused to an amine and aromatic ring
- History: Arylcyclohexylamines were originally developed by Parke Davis for use as anesthetics
- Common Examples: PCP and ketamine are the most common arylcyclohexylamines, but there are hundreds of others
- Legality: Only ketamine is approved for use in medicine. Most arylcyclohexylamines are classified as research chemicals or designer drugs
- Effect Profile: Arylcyclohexylamines are dissociative psychedelics, stimulants, and anesthetics
- Mechanism of Action: Arylcyclohexylamines primarily work by blocking NMDA receptors. They also activate mu-, delta-, and kappa-opioid receptors, sigma-1 and sigma-2 receptors, and inhibit dopamine, serotonin, and norepinephrine transporters as well as nicotinic acetylcholine receptors (nACh) receptors
What Are Arylcyclohexylamine Psychedelics?
Arylcyclohexylamines (AKA arylcyclohexan-amines) are a class of dissociative drugs that decouple one’s first-person, conscious experience from objective reality. They isolate self-aware aspects of the mind from sensory and emotional processing. Users remain fully conscious but lose all physical and emotional “noise” produced by the unconscious mind and physical body.
In lower doses, this produces a sensation similar to alcohol in which one feels as though they’re floating or on autopilot (while paradoxically remaining in control of their body). Euphoria and trippy open and closed-eye visuals are also common.
In higher doses, dissociatives produce profoundly altered states of consciousness in which the user experiences conditions that are hard to describe using spoken language. Users often describe watching themselves without the use of a mirror, falling into an endless abyss, a (non-threatening) confrontation with death, or a merging with the universe.
These drugs work by antagonizing (inhibiting) a receptor called NMDAR (N-methyl-D-aspartate receptor) in the brain. NMDA receptors act as critical regulators of brain activities ranging from memory formation and neuroplasticity to motor function and pain perception. This is the primary mechanism of action for the dissociative action. Most of these drugs also interact with dopamine, serotonin, norepinephrine, and acetylcholine — which leads to further changes to cognition and conscious experience.
The effects of these drugs are a bit of a paradox — they’re both stimulating and sedative; they’re neuroprotective and neurotoxic, and they’re addictive and non-addictive. It all comes down to the dose.
The arylcyclohexylamine class is large and diverse, with most of them having only been invented in the past 10–20 years. The first compound to be approved was PCP (phencyclidine) in the 1950s, but it was replaced ten years later by ketamine which is considered safer and more reliable.
The new wave of arylcyclohexylamine development arose as a result of a sudden surge in public interest for “legal” PCP and ketamine alternatives and designer drugs. Clandestine manufacturers would modify the base structure of PCP and ketamine to produce new psychedelics. The motivation for this spurge in development was to avoid prohibition.
Arylcyclohexylamine Chemistry
The arylcyclohexylamine scaffold contains three regions: an aromatic ring, a cyclohexane ring substitution, and an amine group.
The “aryl-” refers to the presence of an aromatic ring (such as benzene or phenyl). This is one of the most stable chemical structures possible. It’s a feature found in many psychoactive molecules, including benzodiazepines, 2CX compounds, and indole alkaloids like psilocybin and DMT (dimethyltryptamine).
The “-cyclohexyl-” implies the presence of a cyclohexane molecule — a separate six-carbon ring structure forming a hexagon. This group is needed to classify a compound as an arylcyclohexylamine and it appears this group is necessary for the distinct dissociative effect. Whenever this group is removed, researchers note a substantial drop in activity at the NMDA receptors.
The final segment, “-amine” refers to the inclusion of an organic nitrogen-containing molecule.
Reviews
There are no reviews yet.